The Four Phases of Intravenous (IV) Fluid Therapy: The ROSE Concept
IV Fluids are drugs
Although often perceived as such, IV fluids are not just harmless bags of water – they are medications with specific indications and contraindications, and the potential for patient complications and adverse effects.1,2
- Like any other drug, dosing, selection, and timing should depend on the patient’s condition and treatment objectives1
- Healthcare professionals often possess suboptimal knowledge regarding IV fluids and therefore they are frequently used irrationally3
- IV fluids are the most commonly prescribed drugs in healthcare4
- Personalising IV fluid therapy to the patient’s needs can ensure that it remains therapeutic rather than a risk factor5
- The choice of IV fluid can be foundational to patient recovery it can directly shape patient outcomes, from haemodynamic stability to long term recovery6
In this post, we explore the Four Phases of the ROSE concept (Resuscitation, Optimisation, Stabilisation, Evacuation) – a structured approach that can support clinicians in being more intentional with their IV fluid therapy choices.
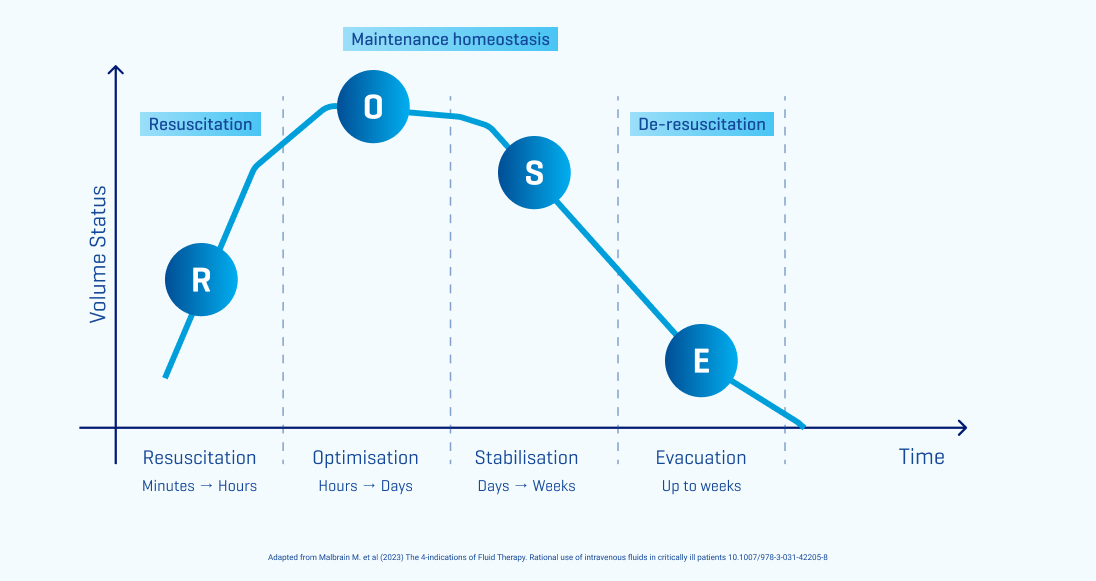
The ROSE concept: A Structured Approach to Fluid Therapy
The guiding principles of ROSE offer a practical, stepwise strategy for the prescribing and administering of IV fluid therapy. Each distinct phase of the ROSE concept corresponds to a different clinical stage in a patient's journey, each with specific objectives to guide appropriate IV fluid and dosage selection to achieve the desired patient outcome at each stage. Understanding these objectives is crucial to ensure safe and effective fluid management:2
Resuscitation: Life-saving phase with focus on patient rescue
Optimisation: Focus on organ rescue and avoiding fluid overload
Stabilisation: Achieve maintenance homeostasis
Evacuation: Focus on organ recovery and resolving any fluid overload
 |
Take a Moment to Stop and Think
Fluid therapy is not static but dynamic taking a moment to stop and think to consider which of the Four Phases your patient is in may lead to a more informed and effective IV fluid therapy choice.
Considering the 6Ds, in analogy with antibiotic therapy, can help at this stage:2
- Diagnosis: depending on underlying conditions, different types of fluids need to be administered
- Drug: approach an IV fluid the same way as any other drug selecting the most appropriate fluid type for the patient’s condition
- Dose: should be adjusted depending on the condition and indication
- Duration: stop IV fluids when they are no longer needed the best fluid may well be the one that was not used unnecessarily
- De escalation: taper fluids when oral intake has resumed and remove excess fluids in case of fluid overload with diuretics or ultrafiltration
- Discharge: when the patient is able to cover their daily fluid need
 |
Fluid Stewardship - A Call to Action
Considering guiding principles such as the ROSE Concept and the 6Ds can help you be more intentional with fluid prescribing and/or administering, however, a more comprehensive approach to fluid management is required to make real change in your organisation.
Fluid stewardship is a coordinated approach to IV fluid therapy. Key principles include:
- Promoting a culture of safety and accountability1
- Continuous education on the importance of IV fluid management1
- Multi disciplinary collaboration with a collective vision3
- Hospital-wide policies on IV fluid prescribing, storage and administration3
- Accountability, assessment and audit3
- Reducing healthcare costs without adversely impacting the quality of care4
By embedding fluid stewardship into practice, healthcare teams can transform IV fluid therapy into an intentional, high impact intervention improving patient outcomes, preventing complications and ensuring the rational use of fluids in clinical practice.1
But how do you practically implement these changes in your daily clinical environment? Contact the Baxter Team.
 |
Learn how your organisation can optimise IV fluid therapy |
References:
1. Malbrain M. (2025) Fluid prescribing and stewardship: strategies to achieve best practice. Hospital Pharmacy Europe. Available at: https://www.hospitalpharmacyeurope.com/wp-content/uploads/trackedfiles/1741945106-baxter-fluid-stewardship-online-book-lr.pdf (Accessed Mar 2025).
2. Malbrain M. et al (2023) The 4 indications of Fluid Therapy. Rational use of intravenous fluids in critically ill patients 10.1007/978 3 031 42205 8s
3. Wuyts S. (2025) Defining fluid stewardship: a hospital pharmacy perspective. Hospital Pharmacy Europe. Available at: https:// www.hospitalpharmacyeurope.com/wp-content/uploads/trackedfiles/1741945106-baxter-fluid-stewardship-online-book-lr.pdf (Accessed Mar 2025).
4. Wong A. et al (2023) Introduction to Fluid Stewardship. Rational use of intravenous fluids in critically ill patients 10.1007/978 3 031 42205 8.
5. Malbrain, M. et al. Intravenous fluid therapy in the perioperative and critical care setting: Executive summary of the International Fluid Academy (IFA). Ann. Intensive Care 10, 64 (2020). https://doi.org/10.1186/s13613-020-00679-3s.
6. Pedder, Andrew et al. Fluid management. Surgery Oxford International Edition, Volume 43, Issue 4, 181 – 189.
Prescribing Information for 0.9% Sodium Chloride (UK & ROI)
Adverse Events and any drug or medical device product quality complaints (including suspected defective medicines or medical device adverse incidents) should be reported.
- For the UK reporting forms and information can be found at www.mhra.gov.uk/yellowcard.
- For Ireland report to the Health Products Regulatory Authority (HPRA) using a Yellow Card obtained from the HPRA, via the online system (www.hpra.ie) or by telephone on +353 (0)1-6764971.
- Adverse Events relating to Baxter products can also be reported direct to Baxter Pharmacovigilance on +44 (0) 800 0260920, or by email to [email protected].
- Drug or medical device product quality complaints relating to Baxter products can be reported directly to Baxter Healthcare Ltd:
- In the UK +44 (0)1604 704603, or by email to [email protected].
- In Ireland on +353 (0)1 2065500 or by email to [email protected].
- Alternatively please report directly to your Baxter Representative, who will take the details and forward to the Baxter Country Quality Assurance Team.